The easiest COVID-19 test there is.
So easy, it’s #1 for families.

Why is InteliSwab® the easy choice?
-
Simple All-in-One Swab & Test Device
-
Only 1 Minute of
Hands-on Time -
Designed and Developed in the U.S.A.
-
 No drops to measure
No drops to measure
For Personal Use

For Healthcare Professionals

For Schools

For Businesses

Results in three simple steps:
SWAB.

SWIRL.

STATUS.
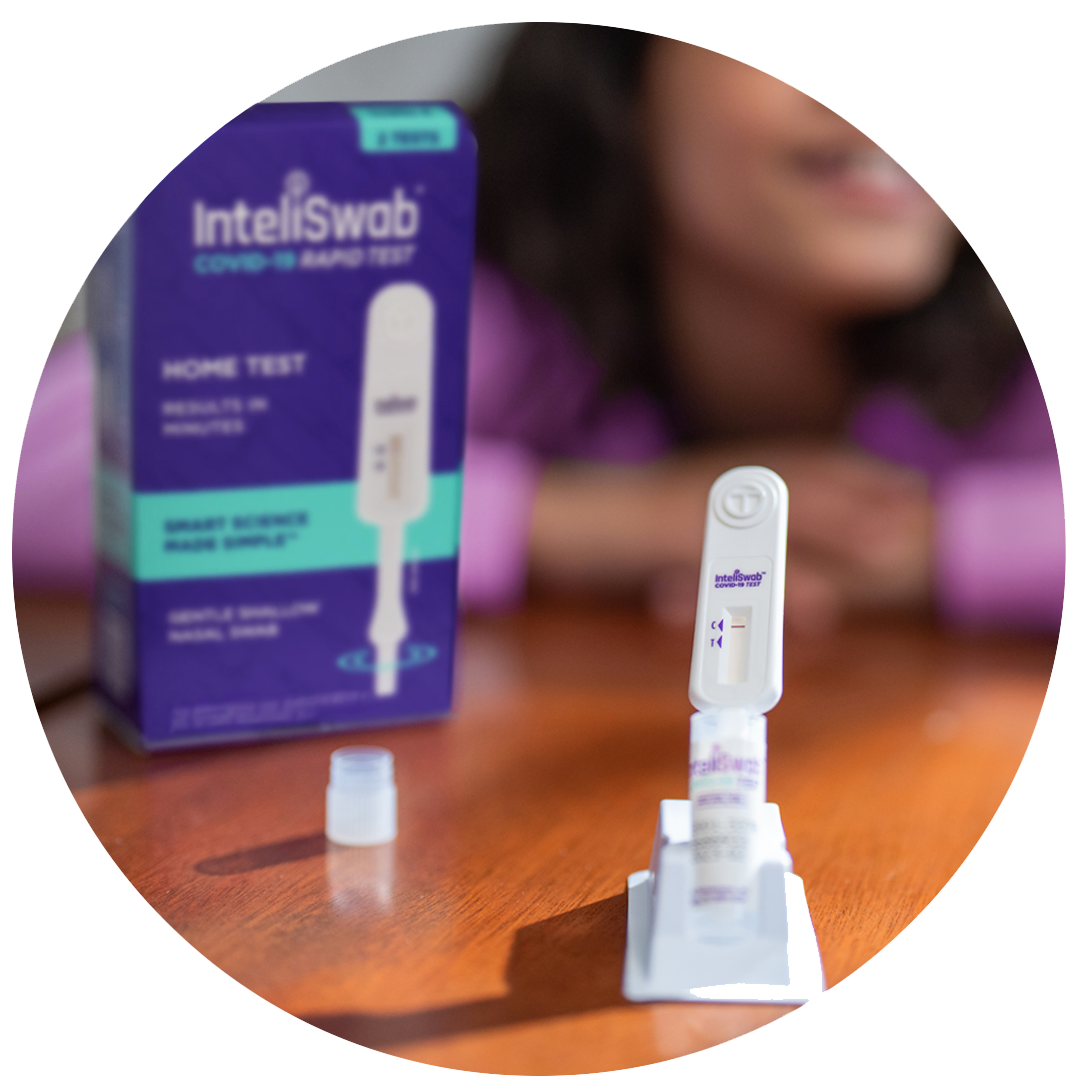
#1 For Families
Featured In:

Former Pennsylvania Health Secretary Rachel Levine refers to [InteliSwab®] tests as “a game changer” in the fight
against
COVID-19.
“[OraSure Technologies’] efforts were praised by President Joe Biden during a stop in the Lehigh Valley last summer.”

Designed and developed in the U.S.A. by OraSure Technologies.
For over 30 years, the innovators at OraSure have worked with the FDA to deliver critical diagnostic tests that protect public health.

Selected by the Department of Health and Human Services.
InteliSwab® is just one of two COVID-19 tests selected by the U.S. government to supply schools nationwide.



